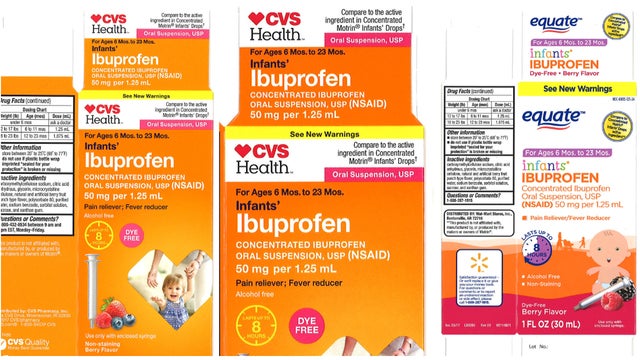
Recall expands for infant liquid ibuprofen sold at CVS, Walmart
WASHINGTON (WCMH) — More infant liquid ibuprofen sold at CVS and Walmart has been recalled over worries they may contain dangerously high concentrations of ibuprofen.
According to the FDA, Monmouth Junction, NJ, Tris Pharma, Inc. is expanding the scope of its November 2018 recall by adding three additional lots of Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 mL, to the retail level.
Some units from these batches have been found to have up to 10 percent above the specified limit of Ibuprofen concentration.
The following medicines are included in the recall:
| Lot No. | NDC | EXPIRATION | DESCRIPTION | COMPANY |
|---|---|---|---|---|
| 4718 | 59779-925-23 | 12/19 | CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 0.5 oz. bottle | CVS Pharmacy |
| 00717005A | 49035-125-24 | 02/19 | Equate: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle | Wal-Mart Stores Inc. |
| 00717006A | 59779-925-24
(Labeled as: 50428-1252-4) |
02/19 | CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle | CVS Pharmacy |
| 00717009A (Previously announced) |
49035-125-23 | 02/19 | Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle | Wal-Mart Stores Inc |
| 00717015A (Previously announced) |
49035-125-23 | 04/19 | Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle | Wal-Mart Stores Inc |
| 00717024A (Previously announced) |
49035-125-23 | 08/19 | Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle | Wal-Mart Stores Inc |
| 59779-925-23 | CVS Health: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle |
CVS Pharmacy | ||
| 55319-250-23 | Family Wellness: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle |
Family Dollar Services Inc. |
Consumers with questions regarding this recall may contact Tris Customer Service at 732-940-0358 or via email at micc_tris@vigilarebp.com.
Consumers, who may be concerned, should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.